

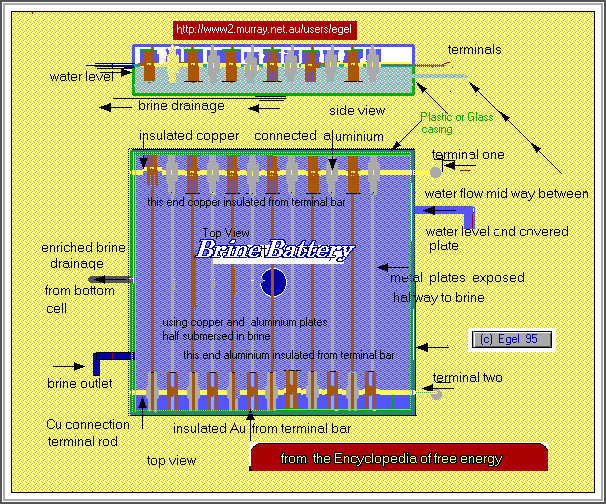
Scrap metal battery
Salt dissolved in water with two strips of different metals can be used to make a battery.
The reaction that causes this flow of electricity dissolves one of the metals and then plates it on the other electrode.
The metals that could be used are copper ,iron,tin, zinc and aluminium A scrap battery could be made using any two these metals by placing the differing metals in alternate rows.
Suspend the metals from insulated supports into the brine solutions. It is recommended that copper be one of the metals used in your battery.
Use salt to water in the ratio 10:1 that is 5 pounds of water by weight to 1/2 pound of salt.
Attach a wire to each electrode and wire either as series connection or parallel connection.
Each metal pair combination will give approx .5 of volt, the current level will depend on the plate area.
Alternate use not illustrated
Unit employing the electro gravitational desalination of saline water U.S. patent no 3,474,014
The invention is owned by General Marine Technology Corporation the address is unknown maybe someone can help me.
They seem to encourage experimenters to build their own units but would get upset if built for commercial profit
In this application several combinations of copper tubing and aluminium rods cells are placed standing upright.
Each cell is separated from one another and has an inlet some where near the bottom of the copper tube length
A area is provided to collect the increase brine concentration at bottom of each tube cell.
A water outlet is provided on opposite side of cell in the copper tube near the top and fed to the bottom of the next one.
Above this is an electrical connection between the copper tube and aluminum provided by a 10 ohm resistor and clear of the brine.
For a hundred gallons a day unit the following are required, a scaled down unit would produced less fresh water and current.
copper tubing 1" diameter X.03 wall by 64 inches long
660 required 3,520 feet in total.
Aluminium rod of same length 1/2 inch diameter use P.V.C. tubing to provide connections between cells
A means needs to be provided to remove the brine from bottom of each tube cell at periodic times when there is a build up of high concentration of salt crystals.
This means opening tubes at the bottom for a fraction of a second while the unit is still running to remove the salt build up.
There are 30 rows of cells and 23 cells to the row
Water flows from the bottom to top in each cell one after the other.
If you want to replace the 10 ohm resistors connect each cell in series so that extra voltage is added from each cell
The electrical circuit must be completed for this unit to work and why not a motor.
From information obtained the average total of 996 watts would be available so this should be enough to drive a small water pump of 1/4 to 1/3 hp to supply filtered sea water or bore water to the unit.
The aluminium rod is fixed inside copper tube with 1/4" space all around to allow the water freedom to flow out of the bottom and the top and inside between the copper tube and aluminium rod.
Brine is fed very slowing from the bottom of the copper tubing, and an electro galvanic response takes place where in simple terms the salt ions are send to the bottom outlet zone.
Meanwhile because there is a flow of water the lesser dense salty brine is carried out to the top and fed to bottom section of another cell and so on until water becomes pure.
Power supply is directly related to the desalination activity and from time to time the aluminium rods will become coated with hydroxides these can be removed by use of a vibrator on each aluminium rod to shake the hydroxides loose.
Care should be exercised during the vibration exercise as there is the danger of an electrical short or an electrocution.
The minimum flow rate should not be less than 10% of the optimum flow rates in units over 10 gallons.
This unit functions not only as a water desalinator but as power source as well.
Babylon battery
In the early history of what is now as Iraq then know as Babylon there is evidence that they knew how to make batteries.
Researchers found a small clay pot with two differnt metal pieces in it and could not work out what it did.
Until one of them poured some vingar in it and connected leads to a multimeter and found it gave a current.
These people certainly knew how to make wine and some must have turned to vinegar and they also knew how to work metals in their weapons.
It is suggested it was kept secret by the early citizens of Iraq and those in the know, who used it to electroplate items to look like gold and then they passed them off as the real thing to early tourists. A real good money earner for the time.
