
When the word hydrogen is mentioned, most people have visions of a pre world war two air ship going up in flames over the United States. Most people think they saw explosions in the newsreel footage but what really happened, was it just burnt to pieces and burnt upwards as well. In the news services there have been claims of inventors making hydrogen from water economically. We shall see in time, if it can be bought forth by them. An automobile suitably modified could be made to run on hydrogen and that hydrogen could be made from the one thing that is in abundance now, sea water, if it could be done more efficiently than today. Getting hydrogen and oxygen is just a simple matter of putting two electrodes in water, keeping them apart and turning on a direct current power source of in excess of 2.5 volts. You should now see two electrode giving off bubbles the faster one is the hydrogen and the other is oxygen. Water that has a 30% caustic soda content could improve the generation
WARNING But be careful when dealing with caustic soda, it can leave a very nasty burn if splashed on your skin, Wash it off immediately upon contact Use safety protection gear rubber gloves and goggles you just can't be too careful when dealing with this substance.
If you don't want to chance it. my informant suggested using washing soda instead.
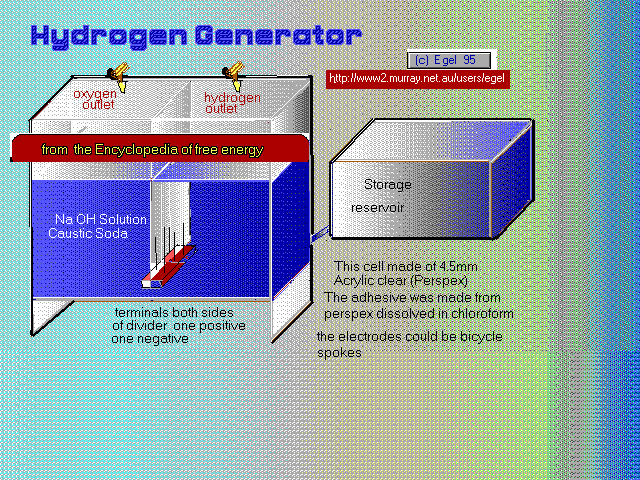
The colour illustration is something along the same lines as above , with some important differences. Two set of electrodes are mounted next to a perspex sheet one side for the hydrogen and the other for oxygen. The electrodes should be set at a height that the gases do not intermingle. By doing this there is formed two collection cells in the device. The water solution is able to pass underneath to both sides, and is filled from an opened topped reservoir set higher than the level used in the reaction chambers ,this is important in control of the gas pressure build up and prevention of an explosion. When electrical power is turned on gas flows from each set of electrodes and into the top of their individual cells. When gas builds up the water levels will drop and water is forced back into water reservoir. The levels will return to normal when gas is removed form the holding cells Gas can be extracted from taps at top of each cells. The electrodes could old carbon rods from spent batteries or iron nails or bicycle spokes. The more in each set of electrodes and closer the better will result in better gas production. The power source could be the brine battery as described in this book as long as voltage is more then 3 volts. A slight alteration could be to use a variable dc power pulse and find by experimentation the best resonance frequency for the gas production. The cells are made from about 4.5mm acrylic clear perspex and can be any size but roughly the same shape as in the illustration. The glue can be made by dissolving some of the perspex in chloroform. Other parts taps ect you should be able to obtain from a good scientific supply house. If you have proper tanks to store your gases they can be recombined in a fuel cell such as those used by NASA to give electricity and pure water at a later date.
