It was observed that the absolute threshold of hearing could be obtained at 0.16Mw (rms), and by calculation that there was an amplitude of displacement of the eardrum of the order of 10 to- eleventh power and a corresponding amplitude of the cochlear basilar membrane of 10 to power - 13 meter. Corollary to this finding. I was able to achieve the absolute reversible threshold of electrolysis at a power level of 0.16 mW (rms).
By carrying out new calculations I was able to show that the water was being vibrated with a displacement of the order of 1 A = 10 to the power of -10 meters. This displacement is of the order of the diameter of the hydrogen atom. Thus it is possible that the acoustic phonons generated by audio side bands of the carrier are able to vibrate particle structures within the unit water tetrahedron.
* * * * * * * *
We now turn to the measurement problem with respect to efficiency of electrolysis.
There are four means that can be used to measure the reactant product of water electrolysis .
For simple volume measurements one can use a precision nitro-meter such as the Pregl type.
For both volume and quantitative analysis one can use the gas chromatography with thermal conductivity detector.
For a continuous flow analysis of both volume and gas species the mass spectrometer is very useful.
Fore pure thermodynamic measurements the calorimeter is useful.
In our measurements , all four methods were examined, and it was found that the mass spectrometer gave the
most flexibility and the greatest precision . In the next section we will describe our measurement using the mass spectrometer.
4 Methodology for the evaluation of the efficiency of water decomposition by means of alternating current electrolysis.
All systems used today for the electrolysis of water into hydrogen as fuel, and oxygen as oxidant apply direct current to a strong electrolyte solution. These systems range in efficiency from 50% to 71%. The calculation of energy efficiency in electrolysis is defined as follows:
" The energy efficiency is the ration of the energy released from the electrolysis products formed ( when they are subsequently used) to the energy required to effect electrolysis."
(1)
The energy released by the exergonic process under standard conditions.
H2(g)+ (1/2)O2(g) ------> H20(1) - 3 02.375 Btu which (1)
is 68.315 Kcal/mol. or, 286,021 Joules/mol, and is numerically equal to the enthalphy charge (triangle H )
for the indicated process.
On the other hand the minimum energy ( or useful work input) required at constant temperature and pressure for electrolysis equals the Gibbs free energy change ( triangle G) 2
Penner shows (Op.Cit.) that there is a basic relation derivable from the first and second laws of thermodynamics for isothermal changes which shows that
triangle G = triangle H - T triangle S
where triangle S represents the entropy change for the chemical reaction and T is the absolute temperature.
The Gibbs free energy change ( triangle G) is also related to the voltage (e) required to implement electrolysis by Faraday's equation,
e=( triangle G/23.06 n ) volts
Where triangle G is in Kcal/mol, and n is the number of electrons( or equivalents ) per mole of water electrolysed and has the numerical value 2 in the equation, ( endergonic process)
Therefore, according to equation (2) at atmospheric pressure, and 300 degrees K , triangle H=68.315 kcal/mol or h2O (1), and triangle G = 56.620 kcal / mol of H2O (1) = 236,954 J/mol H20 (1) for the electrolysis of liquid water.
In view of these thermodynamic parameters for the electrolysis of water into gases, hydrogen and oxygen, we can establish by Eq.(2) numeric values where,
triangle G (J/mol)
n= ------------------------- =<1
triangle G e (J/mol)
where triangle G e is the electrical energy input to h2O (1) in Joules,
and triangle G is the Gibbs free energy of H2O (1) . The conversion between the two quantities is One Watt second (Ws) = one Joule.
Or, in terms of gas volume, as hydrogen ,produced and measured ,
measured H2 (c.c)
n= ---------------------- =<1
Ideal h2 (c.c)
in accordance with these general principles we present the methodology followed in evaluating the electrolytic of alternating current on H2O (1) in producing the gases, hydrogen and oxygen. No attempt has been made to utilize these gases according to the process of Eq(1).
It is to be noted that the process
yields only 57.796 kcal /mol. Eq (7) shows that per mole of gases water formed at 300 degrees K, the heat released is reduced from the 68.315 kcal/mol at Eq. (1) by the molar heat of evaporation of water at 300 degrees K (10.5 kcal) and the overall heat release is 57.796 kcal/mol if H2O (G) is formed at 300 degrees K
In the following sections we describe the new method of electrolysis by means of alternating current, and the exact method and means used to measure the endergonic process of Eq.(4) and the governing Eq.(2) and Eq. (15)
1. Op.Cit. Ref. (1) page 3. page 299ff.
In order to properly couple Component II to a mass spectrometer one requires a special housing around Component II that it will capture the gases produced and permit these to to be drawn under low vacuum into the mass spectometer. Therefore a stainless steel and glass chamber was built to contain Component II,and provision made to coiuple it directly through a CO2 watertrap to ther mass spectrometer and Component IV were purged with helum evacuated for a two hour period before any gas sample were drawn.In this way contanination was minimized, The definitive measurement were done at Gollob Analytical Services,Inc in Berkeley Heights ,new Jersey
We now describe the use of Component I and how it energy output to Component II is measured. The energy output of Component I is an amplitude modulated alternating current looking into a highly non-linear load, i.e ,The water solutiuon Component I is so designed that at peak load it is in resonance across the sysytem - Components I,II, and III , and the vector diagrams show that the capacitive reactance,and the inductance reactance are almost exactly 180 deg out of phase,so that the net power output is reactive ( the dissipative power is very small).This design insures minimum power lossses across the entire output system.
In the experiments to be described,the entire emphasis is placed on achieving the maximum gas yield (credit) in exchange for the minimum applied electrical energy, The most precise way to measure the applied energy from Component I to Component II and Component III is to measure The power, P, in watts,W. Ideally this should be done with a precision wattmeter. But since we were interested in following the voltage and current separately,it was decided not to use the watt meter. Separate meters were used to continuously monitor the current and the volts,
This is done by precision measurement of the volts across Component III as root mean square (rms) volts;and the current flowing in the system as rms amperes.Precisely calibrated instruments were used to take these two measurements. A typical set of experiments [ using water in the form of 0.9 saline solution 0.1540 molar] to obtain high efficiency hydrolysis gave the following results.
rms Current = I = 25mA to 38 mA (0.025 A TO 0.038 A.)
rms Volts =E = 4 Volts to 2.6 Volts
The resultant ration between current and voltage is dependent on many factors such as the gap distance between the center and ring electrodes,dielectric properties of the water,conductivity properties of the water,equilibrium states,isothermal conditions,materials used,and even the presuure of clathrates. The above current and voltage values reflect the net effect of various combinations of such parameters.When one takes the product of rms current,and rms volts one has a measure of the power, P in watts
P= I x E = 25 mA x 4.0 volts =100 mW (0.1 W)
and P = I x E =38uA x 2.6 volts = 98.8 mW (0.0988 W)
At these power levels (with load), the resonant frequency of the system is 600 Hz (plus or minus 5 Hz) as measured on a precision frequency counter. The wave form was monitored for harmonic content on an oscilloscope, and the nuclear magnetic relaxation cycle was monitored on an X-Y plotting oscilloscope in order to maintain the proper hysteresis loop figure. All experiments were run so that the power iin watts, aplied through Components I,II, and III ranged between 98.8 mW to 100mW.
Since by the International System of Units -1971 (ST),
One Watt-second (Ws) is exactly equal to one Joule (J),our measurements of efficiency used these two yardsticks (1 Ws = 1J) from the debit side of the measurement.
The energy output of the system is,of course, the two gases,Hydrogen(H2) and Oxygen (1/2O), and this credit side was measured in two laboratories,on two kinds of calibrated Instruments, namely
Gas Chromatography Machine, and, Mass Spectrometer Machine.
The volume of gases H2 and (1/2)O2 was measured as produced under standard conditions of temperature and pressure in unit time,i.e, in cubic centimeters per minute (cc/nin), as well as the possibility contaminating gases,such as air oxygen, nitrogen and argo, carbon momoxide, carbon dioxide, water vapor etc.
The electrical and gas measurements were reduced to the common denominator of Joules of energy so that the efficiency accounting could all be handled in one currency. We now present the averaged results from many experiments. The standard error between different samples,machines,and locations is at minus or plus 10 percent and we only use the mean for all the following calculations.
II. Thermodynamic Efficiency for the Endergonic Decomposition of liquid water (salininized) to gases under standard atmosphere (754 to 750 m.m. Hg)' and standard Isothermal conditions @ 25 deg C = 77 deg F = 298.16 deg K, according to the following reaction.
H20 (1) _> h2(g) + (1/2)O2(1) + triangle G 56.620 Kcal /mole (10)
As already described triangle G is the Gibbs function.
we convert Kcal to our common currency of Joules by the formula
One Calorie =4.1868 Joules
Triangle G = 56.620 Kcal x 4.1868 J = 236,954/J/mol of H2) where
1 mole =18 gms.
Triangle G2 the electrical energy required to yield an equivalent amount of energy from H2O in the form of gases H2 and (1/2)O2.
To simplify our calcualtion we wish to find out how much energy is required to produce the 1.0 c.c. of H2O as the gases H2 and (1/2)02.
There are (under standard conditions ) 22,400 c.c. - V of gas in one mole of H2O. Therefore
triangle G 236,954 j
-------- = --------- = 10.5783 j/cc
V 22,400 cc
We now calculate how much electrical energy is required to liberate 1.0 cc of the H2O gases (where H2 = 0.666 parts, and (1/2)O2 = 0.333 parts by volume ) from liquid water. Since P= 1 Ws= 1 Joule , and V= 1.0 cc of gas = 10P:5783 Joules, then
PV =1 Js x 10.5783 J = 10.5783 Js or
=10.5783 Ws
Since our experiments were run at 100 mW( 0.1 W) applied to the water sample in Component II,III, for 30 minutes ,we wish to calculate the ideal (100% efficient) gas production at this total applied poer level. This is, 0.1Ws x 60 sec x 30 min =180,00 Joules ( for 30 min )
The total gas production at Ideal 100% efficiency is 180.00 J/10.5783 J/cc =17.01 cc H2O (g)
We further wish to calculate how much hydrogen is present in the 17.01 cc H2O (g).
17.01 cc H2O (g) x 0.666 H2 (g) = 11.329 cc H2(g)
17.01 cc H2O (g) x 0.333(1/2) C2 (g) = 5.681 cc (1/2)O2 (g)
Against this ideal standard of efficiency of expected gas production ,we must measure the actual amount of gas produced under
1) Standard conditions as defined above. 2) 0.1 Ws power applied over 30 minutes. In our experiments , the mean amount of H2 and (1/2)O2 produced ,as measured on precision calibrated GC, and MS machines in two different Laboratories, wher S.E is plus or minus 10%
Measured Mean = 10.80 cc H2 (g)
Measured Mean - 5.40 cc (1/2) cc (1/2) O2 (g)
Total Mean 16.20 cc H2O (g)
The ratio N1 between the ideal yield, and measured yield,
Measured H2 (g) 10.80 cc
N1 = --------------------- ----------- 91.30%
Ideal H2 (g) 11.33 cc
This method is based on the number of electrons that must be removed, or added decompose ,or form one mole of, a substance of valence one. In water H2O,one mole has the following weight:
H= 1.008 gm /mol
H= 1.008 gm /mol
O = 15.999 gm/mol
Thus, 1 mol H2O = 18.015 gm/mol
For a unvalent substance one gram mole contains 6.022 x 10 to 23 third power electrons = N = Avogadro's Number. If the substance is divalent,trivalent,etc,. N is multiplied by the number of the valence. Water is generally considered to be of valence two.
At standard (STP) temperature and pressure one mole of a substance contains 22.414 c.c., Where Standard temperature is 273.15 deg k = ) 0 deg C. =T . Standard Pressure is One atmosphere =760 mm.Hg =P
One Faraday (1F) is 96,485 Coulombs per mole (univalent).
One Coulomb is defined as:
1N 6.122 x 10 to the 23 power Electrons
------- = --------------------------------------------- One Coulomb
1F 96.485 Coulombs
the flow of one coulomb per second = One Ampere
One Coulomb x One volt = one Joule second
In alternating current when amps (I) and Volts (E) are expressed iin root mean squares (rms) their product is Power.
P= IE watts
With these basic definitions we can now calculate efficiency of electrolysis of water by the method of Faraday is electrochemistry.
The two-electron model of water requires 2 moles of electrons for electrolysis (2x6..022 x 10 to the 23 power),or two Faraday quantities (2x 96,485 = 192,970 c.).
The amount of gas produced will be:
H2 = 22,414 c.c. /mol at STP
1/2O2 = 11,207 c.c. / mol at STP
Gases = 33.621 c.c. /mol H2O (g)
The number of coulombs required to produce one c.c of gases by electrolysis of water
193,970 C.
-------------------- = 5.739567 Coulombs per c.c. gases
33,621 c.c
Then 5,739 Coul /cc /sec =5.739 amp/sec/cc.
How many cc of total gases will be produced by 1 A/sec?
0.1742291709 cc.
How many cc of total gases will be produced by 1 A/min ?
What does this represent as the gases H2 and O2 ?`
1/2C2 =3.136438721 cc / Amp/min.
H2 =6.2728 cc/Amp /min
We can now develop a Table for values of current used in some of our experiments,and disregarding the voltage as is done conventionally.
I calculations for 100 mA per minute
Total Gases 1.04537 cc/mim
H2 = .6968 cc /min
1/2O2 = .3484 cc/min
30 min. H2 =20.9054 cc/ 30 minutes
II Calculations for 38 mA per minute
Total Gases = 0.3972 cc/ 30 minutes
H2 = 0.2645 cc/ min
1/2O2 = 0.1323 cc/min
30 min. H2 7.9369 cc/min
III Calculations for 25mA per minute
30 min. H2 = 5.2263 cc/ minute

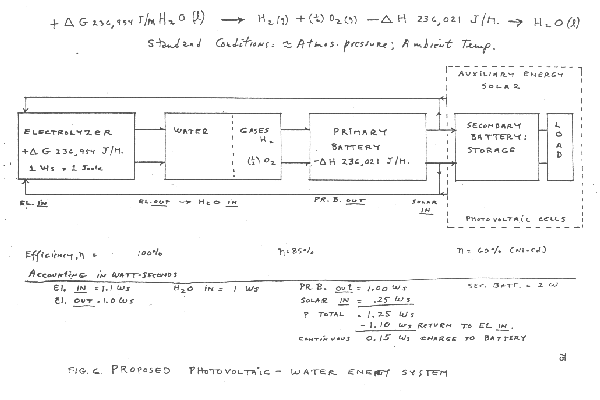
Figure 6 and 7 show two of the many energy production systems that may be configured to include renewable sources and the present electrolysis technique. Figure 6 shows a prposed photovoltaic powered system using a fuel cell as the primary battery. Assuming optimum operating conditions using .25 watt seconds of energy from the photovoltaic array would enable .15 watt seconds to be load.
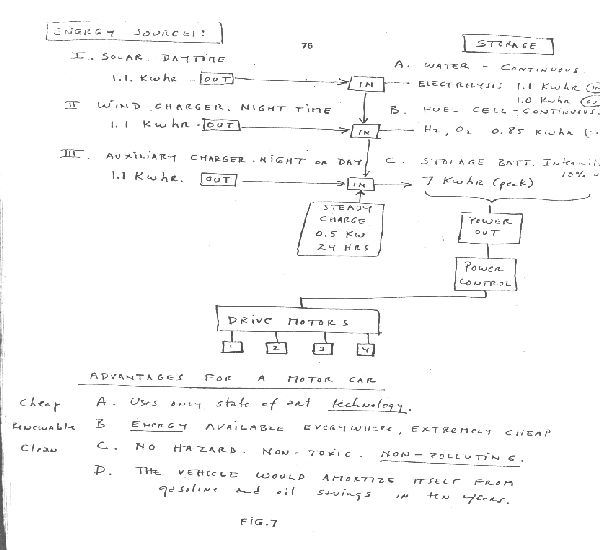
Figure 7 depicts several renewable sources operating in conjuncction with the electrolysis device to provide motive power for an automobile.
Text Supplied and original diagrams From Fred Epps Thanks
Some drawings have been reproduced by Geoff Egel

back to Puharich Papers part one
