

Part one / Part two / Part four / Part five appendix
(2)EFFECT OF PLATE AREA ON OUTPUT CURRENT
It was realised that the output current should vary directly with the plate area for a constant plate separation distance. The curve in Figure 12 shows the effect of plate area on the output current for a constant dosage rate and .050" plate separation distance. The curve shows that the output current varies directly with the plate area as expected.
At this point it was realised that using contact potential in an ionisation chamber would not result in a better field instrument. For a practical instrument, additional amplification would be required since sensitive galvanometers are bulky. unstable and very sensitive to shock.
Calculations show that an 8" dia. cyclinder, 10" in length containing concentric cylinders made up of aluminium and copper .3evarated by 050" as shown in Figure 13, could he used with a small field type galvanometer to measure up to 3000 r/hr., -108r/hr/div. This would definitely be too high a range for military use as a survey meter. To obtain a smaller range instrument without having a very large bulky instrument. a very sensitive galvanometer or amplification would have to be used.
P.E. Chmart(17) has developed an instrument using contact potential as the chamber voltage. This instrument is a good laboratory instrument but has no advantage over other instruments for field use. The elimination of a "B" battery by using contact Potential doesn't outweigh the additional expense of the chamber plus the probable increase in energy dependence. Thus, attention was focused on these phenomena to be used as a radioactive battery consisting of cells made up of aluminium and copper, placed in an inert atmosphere with a built-in radioactive source. All of the information obtained up to this point would be needed such a battery. It was realised that so far work had a radioactive cell. It was thought that it should be put these cells in series and obtain a battery of any voltage. An immediate application would be a voltage charge a quartz-fiber dosimeter which requires approximately 16OV and very little current.
(3)EXPERIMENTAL RESULTS OBTAINED FROM PUTTING CELLS IN SERIES
It was thought that it should be possible to put these cells in series to form a battery, The cells of the experimental battery
In developing been done and possible is shown in Figure 13, which consists of concentric cylinders of aluminium and copper foil spaced .050" with Leucite spacers were connected in series and the internal load was measured using the ionisation method NOTE:Plate Size 1/16" thick * 2" x 2"
Figure 14
Results of this test are shown in the following table:
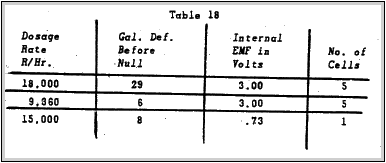
NOTE:Theoretically. if all the cells ere .73V, the internal EMF should have been 3,65 V. The discrepancy is due to the varying cell voltages
(4)RESULTS FROM USING DIFFERENT CASES
Now the question arose as to the type of gas to use in this type of battery. Knowing that the contact potential of metals change an the oxides form in air or when reactions with other gases take place, it was decided that an inert gas should he used. Other gases Could be used providing voltage regulation Is not too critical and the decrease in contact potential not so great that
the physical size becomes a factor. Of course if the gases were not inert some of the-gas would he lost in combining with the metals.
Xenon seemed to be the best inert gas. Its density is about five times that of air, Results have shown that there is approximately 16 times as much ionisation using Xenon an compared to air with .6 MEV X-rays. Tests were conducted .0 find the saturation point' for air and other gases, The cell shown in Figure 6 consisting of the 1.5" al and pt. plates with the al plate movable. was used for this test. The cell was placed in the radiated field of an X-ray machine. A very sensitive galvanometer was-used to measure the current output. Figure 15 shows-the experimental arrangement. Figure 16 shows the output current vs dosage rate in r/hr. for different separation distances between the two metal plates al and pt. with nitrogen and air as the gases. 050" plate separation distance was found to he the optimum distance. The output expected since the density of nitrogen and air is nearly the same but the pressure of the nitrogen was twice as great as that of air. The cell saturated at around 14,000 t/hr. The maximum output current obtained was l.23 x 10-8 amperes.
Figure 17 shown the results of using Argon at different pressures as compared to air and nitrogen. The results 2how that at 48.2 PSIA of Argon gas there was a lot of recombination. Thus. saturation was reached at low dosage rates. The shape of the curve for .050" plate separation distance, Argon gas at 48.2 PSIA in not fully understood since one would expect the curve to rise to the maximum current obtained at 30 PSIA and then remain flat. These curves show what would be expected. that there is an optimum plate Separation distance. The saturation was at approximately 16000 r/hr. The maximum output current obtained was 3.96 *z 10-8 amperes.
(5)PREPARATION OF SURFACE CONDITIONS OF METALS
In all of these experiments the metal plates were prepared as
follows
(1) A grade 92 steel wool was first used to smooth up the surfaces
(2) This surface was further prepared with 00 fine steel wool until a mirror surface was obtained,
(3) A clean cloth as then used to wipe the surface clean after which the plate was ready to be used in a cell.
Some tests were conducted to determine the effects of polishing the metals on the contact potential. The results are shown in the following table:
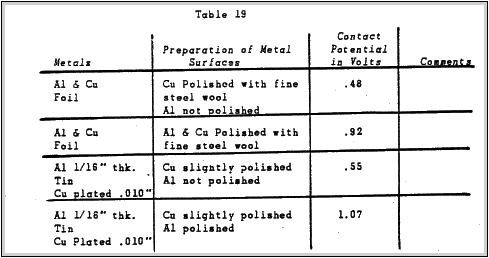
The results show that cleaning and polishing the metal surfaces of both metals increases the contact potential considerably.
(6)EFFECT OF OXIDATIOIN OF METALS ON CONTACT POTENTIAL
Results in the previous discussion show that the contact potential between aluminium and copper is decreased considerably with oxidisation. The question now arose as to what was the rate of decrease. and which metal caused the greatest change. Two plates were polished and left in the air to oxidise. Two other metal plates were polished and put in a desicator since Cu doesn't react with dry air very readily.
The results of this experiment indicate that the oxidation of Al is the larger factor in the decrease of the contact potential due
to oxidation of AI and Ca. A more comprehensive study should be made before this fact -is definitely established.
Leland L. Antes(18) and Normm Hacheraun have made a study of the .initial oxidation process at low pressures in their report. "Contact Potential Variations on Freshly Condensed Metal Films at Low Pressures. " His results also show that the contact potential decreases between AI and Ca when oxidation takes place.- The contact potential between pt and al of our first cell decreased from 0.57 V to 0.46 V in a three months time being exposed to the atmosphere.
DESIGN 4 EXPERIMENTAL RESULTS ON 97 CELL AL & CU. PLATE BATTERY
A design was worked out for a 97 cell battery consisting of al and cu plates,connected in series. Air was to be used as the gaseous medium. The ionising agent was a 250 KV X-ray machine. Figure 18 shows the completed battery consisting of 2" x 2" z 1/16" cell and cu plates stacked in series with each combination of al and cu plate forming a cell being separated by a .050" lucite spacer. ..The results of the tests performed on this battery are shown. in the following table:

