
Radio Active Battery Part 2
Part one / Part three / Part four / Part five appendix
c. FACTORS AFFECTING CONTACT POTENTIAL
(1)METALS
Since the contact potential is equal to the difference in work functions of the metals ,the type of metal has considerable affect on the contact potential or the internal EMF of the radioactive battery.
Of course to obtain the maximum EMF one should use the combination of a very low work function metal and high work function metal. Metal like platinum and iridium have very high work functions but of course, are very expensive.
The alkali metals have very low work functions but are difficult to work with. Thus in designing a battery using contact potential the question of economics has to be considered together with work function. A contact potential of 2.7 V has been observed between sodium and tungsten.
A discussion of our results on the measurement of contact potentials with different metals will be mentioned later in the article.
(2)SURFACE CONDITION OF METALS
The surface condition of the(metals does affect the contact potential. Herbert H. Uhlig (11)in his report. "Volta Potentials of Copper-Nickel Alloys and
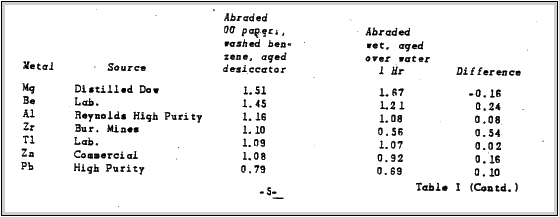
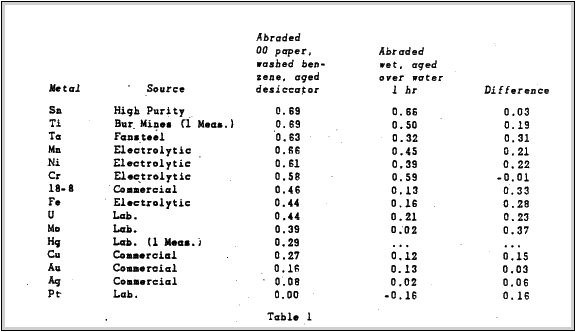
Several Metals in Air. examined this effect quite extensively. Most of his data in his report was obtained for surfaces abraded with numbers 1.0 and 00 emery paper, followed by swabbing with cotton soaked in benzene, and then washing in several beakers of distilled benzene.
For some measurements, specimens were abraded under water using waterproof emery paper 6OOA.These specimens were then washed under the distilled water tap. dried in a stream of air previously passed over Ca Cl2 and immediately measured.
Volta Potential Series in Air vs Pt Volts
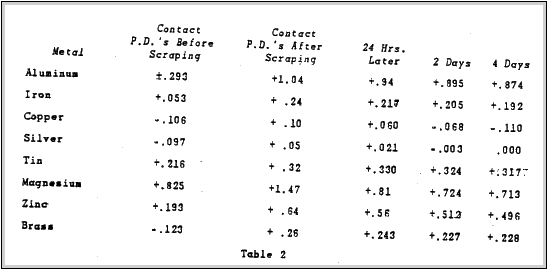
Table 1 taken from Mr. Uhlig's report, lists values obtained for various metals with respect to a platinum reference electrode. The metals were abraded as previously described, finishing with 00 emery paper. Reproducibility of measurements was frequently +.01 Volt but sometimes as poor as +.06 Volt. The second series of measurements obtained for metals abraded under water showed a maximum variation of +1 Volt. A platinum electrode abraded dry an above was again used as a reference. Albert E. Hennings made
a study of the effect of surface preparation of metals upon the
contact potentials. He mentions that the only way to prepare a
pure and approximatelyfilm-free surface of any ordinary metal is to cut away the metal.
Table 2 shows the results of his measurements of contact potential between various metals and brass before and after scraping in a vacuum.
(3)TEMPERATURE
Temperature variations definitely affect the contact potential. between two metals. This variation is not too great. Increasing the temperature of a metal lowers its work function. Since the contact potential between two metals is equal to the difference in work function of the metals. one would expect temperature variation to cause little change in the contact-potential.
Actually temperature changes effect the work function of metal differently so that there is a change in contact potential.
Harry C. Bunbridge (12) made a study of this effect and published the results in the 1913 issue of Physical Review. Figure.5 shown a curve indicating the change in contact potential between copper and the metals shown with a temperature change.
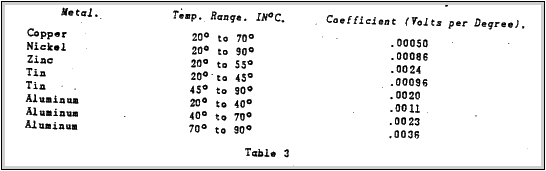
Table 3 shows the temperature coefficients of contact Potential. Note that for aluminium and cooper the change in contact potential .between room temperature 200C and 1200C is only .001.1 volts per degree. Also note that the contact potential increases with an increase in temperature. An investigation of the effect of contact potential at lower temperature should be mode.
(4) PRESSURE
Albert Hennings found that contact potentials of metals wore
not noticeably changed by difference in pressure from atmospheric pressure to the best vacuum obtainable. Table 4 shows the results of contact potential measured at atmospheric pressure and in a vacuum between brass and the metals in-the first column.
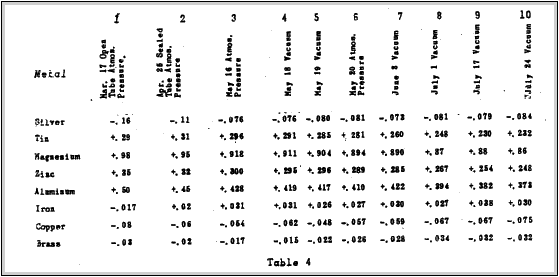
A survey of all the results in Table 4 shows that the contact potentials must he independent of pressure. None of the changes are abrupt. indicating that they are all due to the mere ageing with time. The metals on the whole have become more electro-negative.
This does not appear to be true in all cases, but the apparent discrepancy lies in the fact that the brass plate with which these metals have been compared is also subject to changes.
(5)SUNLIGHT
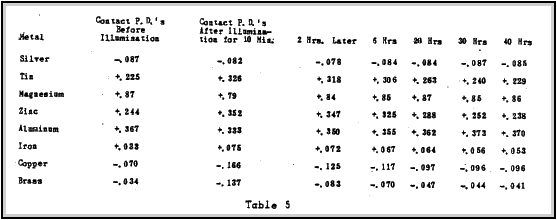
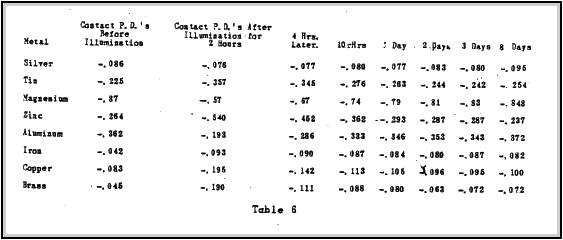
Sunlight has been found to have little effect on the contact potential of two metals. Albert E. Hennings (14) ) made a study of the effect of sunlight on the contact potential of metals.
Tables 5 and 6 show the experimental results obtained after A mercury arc radiating the metals with ultra-violet light.
light fed by a current of 1.85 amperes was placed approximately
20 cm from the 2 cm disks. The data gives the contact potential between brass and the metal listed. This effect can be eliminated in the radioactive battery design by using coloured lucite or glass painted with optical black paint.
d.RADIOACTIVE BATTERY
Thus far this article has discussed contact potential without much
mention of a battery,. Contact potential is the most important consideration in a radioactive battery. 'In a radioactive Battery the contact Potential provides the voltage and the radioactive source produces the ions necessary to obtain a current.
Greinacher(15) in 1905 actually built a radioactive cell. He was interested in measuring the contact potential of two metals. He used a lead strip, on which was deposited a radioactive source, tellurium as one of the plates.
Experimental work was started in the laboratory on the investigation of contact potential with the view in mind of using this potential in an ion chamber for measuring radiation. Realising that there were a number of factors affecting the output current from a cell made up of two metals separated by a gas medium. a cell was constructed that could investigate these factors experimentally. This device is shown in Figure 5. It consists of two metals. 1.5" sq. platinum and aluminium. enclosed in a gas tight lucite chamber with one plate movable to that the plate distance could he adjusted.
The chamber was placed in an X-ray field and measurements were made. A schematic diagram of the test set up is shown in Figure 7.
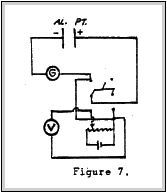
Figure 8 shown a photograph of the experimental arrangement. Figure 9 shows a curve of output current vs dosage rate with an applied voltage of 150 KV on the X-ray machine. The plates were brought in contact and then the aluminium plate was separated until an ohmmeter showed an open circuit. The curve shows that the output current is directly proportional to the incident radiation. A contact potential of .57V was measured.
(1)EFFECT OF DISTANCE BETWEEN PLATES ON OUTPUT CURRENT
Since an increased plate separation distance decreases the field and also increases the effective volume of ions. there is an optimum plate separation distance for obtaining the maximum output current.
Figure 10 shows a curve of galvmometer defelection vs plate separation with a constant radiation


