
Radio Active Battery Part 4
Part one / Part two / Part three / Part five appendix
CALCULATION OF INTERNAL RESISTANCE OF 97 CELL BATTERY

The battery was placed approximately 3" from the X-ray machine. The effective energy of the X-ray:s was .07 Mev The above formula can be used with reasonable accuracy at this energy level.
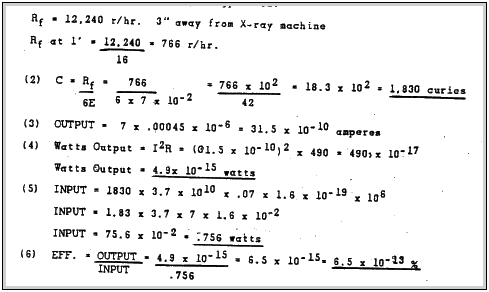
Thus one can see that this type of a battery in very inefficient. The reason for this low efficiency is probably due to the low efficiency of conversion from gamma to ions, absorption of gamma rays in the al and cu and thermal 5 losses.
Since this battery has such a high internal resistance. it is a constant current device until the load resistance in equal to the internal resistance of the battery. Thus the efficiency of a radioactive battery should increase with load until the load is equal to the internal resistance of the battery. For maximum power output in any electrical generator, the external impedance of the equal the internal impedance of the generator. If the load were increased to 10^10 ohms the current would decrease one-half. The output would increase 5 X 10^6. The overall efficiency would he approximately 3.25 x 10^-7%.
This fact should be checked experimentally.
This magnitude of efficiency compares favourably with P. E. Ohmart's(19) work on his cell. He obtained an emf efficiency of .01 percent on a cell using magnesium dioxide and lead-dioxide with argon as the gas and Ag^110 as the radioactive source.
f.RADIOACTIVE BATTERY COMPOSED OF 212 CELLS
The battery previously described was tested using X-rays as the
ionising agent. Work was concentrated on the design of a battery using a radioactive source as the source of ionisation.
RADIOACTIVE SOURCE
A study was made of the main radioactive sources that could be used in a radioactive battery.
(a)GAMMA SOURCE
Gamma sources are not desirable because of the shielding problem. and also since a gamma ray is a neutral particle its absorption is less and thus its Ionising effect on a gas is much lost than for a charged particle.
(b)ALPHA SOURCES
Since the alpha particle is heavier than the beta 13articlo@ the specific ionisation is much greater.. The limiting factors
are:
- large expense and difficulty in obtaining alpha sources.
- short range of the alpha particle.
Most of the long half-life alpha sources are in the radium and thorium series. These sources are quite expensive and difficult to obtain.
The range of alpha particles from radium and thorium is approximately 3.5 cm - 4 cm in air. In an inert gas such as argon the range would be much loss. The range of these alpha particles in aluminium has about the same density as lucite is approximately 10^3 cm
Thus if an alpha source wore to be used in a radioactive battery, it would have to he coated on the surfaces of the plates themselves.
(c)BETA SOURCES
A beta source seems to be the ideal isotope for use in an atomic battery. The charged particle produces a large number of ions. Since the beta particle is a charged there is no shielding problem. A number of beta sources with long half-life are available commercially. Sr 90 with a half-life of approximately 30 years seems to he the best radioactive isotope for use in radioactive batteries. Its cost in very small approximately $1.35/mc.
(2)INERT GASES
Since it-was found that the contact potential of two dissimilar metals-decrease with oxidation. It was decided that an inert gas would have to be used.
A study of the inert gases was made. Since ionisation increases with the density of the gas. an inert gas with the greatest density possible should be used. Xenon was found to be the best gas. Its density is 5.851 g/1 at 0'C. 760 mm pres. approximately 4.5 times that of air.
(3)METALS FOR CELLS
A study was made of the contact potential. Availability Weight and cost of various metals. To obtain the maximum contact potential a combination of the highest work function metal and the lowest work function metal obtainable should be used. In looking at a table of work functions, one can see that platinum has about the highest work function obtainable and the alkali metals are very difficult to work with. The two metals that seem to be best from an all around viewpoint were aluminium and copper.
(4)MECHANICAL DESIGN
Having decided upon the metals source and gases the next problem was the mechanical design of the battery.
In order to decrease the weight and size the plating of cooper on aluminium was tried. Since a brass plating upon zinc that had been plated upon aluminium foil was available, this material was used in the battery. A check of the contact potential between brass and aluminium was made and found to be approximately 0.95 V,
about the same as that measured between copper and aluminium. It was decided to use Lucite as the battery case and cell support, because of its ease in working. Probably in practice metal or glass would he used because of the absorption of gas in lucite after a long time.
The support and arrangement of the metal plates is shown in Figure 19.
Figure 19
INSIDE OF 212 C-ZLL BATTERY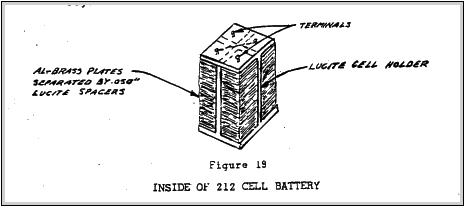
The plate size 1/2" x ½" was determined from a consideration of
size and internal resistance of the battery. The battery was to he used to charge a uuf condenser. The frame plate support in shown in the above sketch. The plates are held in place by a band between the lucite and metal. Rez-N-bond solvent was used to soften the lucite and create the band. The metal plates were separated by small .050" tick. lucite spacers Electrical contact was made by screwing small brass screws down on the top of the plates.
(5)SIZE OF SOURCE
A thallium 204 beta source had to he used because 5r90- was not available. The size of the Thallium 204 source to he used was determined from a consideration of previous experimental results and a check on the activity of the Thallium source.
One tenth mc of Thallium 204 spread over 1 sq. in. had an activity of 6gr/hr. 1/4" away. Test results show that a cell with approx. 1 V contact potential saturates at approximately 16000 r/hr with argon at atmospheric pressure. For Thallium 204. the ionisation with argon in twice as much as the ionisation in air. Thus, saturation in air would he 16OOO x 2 - 32,000 r/HR.

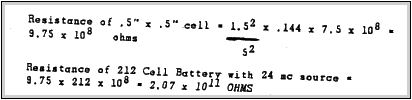
Figure 20 shows the completed battery. The case was made up in two half sections.
An investigation of the shielding showed that .092" of Incite would be adequate shielding for a thallium 204 source. 125" lucite was used for the walls of the battery. The source was deposited on the four sides and a thin plastic coating applied over the source to keep it in place. A metal to lucite seal was developed that showed not leakage at 15 psia. In order to facilitate filling the battery with gas, it was decided to fill the container with a gas pressure of slightly above Atmospheric pressure.
(6)TEST RESULTS
Xenon was allowed to pass through the battery for a short time after the half-sections had been assembled and the top put on. It was hoped that the Xenon would push out cost of the air in passing through. Only a small container of Xenon was available and the gas was used up sooner than anticipated. The exit and entrance holes were capped. completing the battery. Test results on this battery were had. No observable output could be detected. Air probably was in the battery, because the Xenon gas pressure dropped too soon during the filling operation.
The entrance and exit caps were taken off and the battery was filled with argon. Tent results. showed a definite power output. Approximately 1 div. or 4.6 x I0^10 amp was observed. A measurement of the internal EMF showed 70V: This reading was eratic. The next day only 10 V was detected.
4.CONCLUSIONS:
The results obtained to date from an investigation of contact potential and
the utilisation of this physical phonomena in a radioactive battery definitely
indicated that this type battery is useful for the production of small currents.
cat
Numerous applications for such a battery indicate a definite need for farther work in this field.
ADVANTAGES AND DISADVANTAGES OF RADIOACTIVE BATTERIES
ADVANTAGES Relative low cost battery when long shelf life is considered
A very small battery with a high internal emf can he built
A very long shelf life battery
Light weight
Capable of operation at extreme temperature conditions
DISADVANTAGES
Large initial cost
Limitation on power output
Very low power output for
a reasonable size battery
5.ACRNOWLEDGEMENT:
Acknowledgement is given to Mr. Howard Hasolkorn who carried on this
Investigation with the author. Appreciation for efforts given in obtaining data and assistance in construction of the batteries are extended to Richard Daniels. Stanley Grossman and Ann Yan Cleve.
6.REFERENCES:
(1)Ice F. Patal and Martin A. Pomerantz. *%Contact Potential Differencer,", P. 239
(2)A. Cochn. Hand d. Phys. Vol XIII, p. 332 (1928)
(3)E. Perucca. Atti del Congresso Como
(4)A. Sommerfeld. Zoitz, f. Phys. Vol 47, p 1 (1928)
(5)C. Eckart. Zeits f. Phys. Vol 47 p. 38 (1928)
(6)Imre F. Patai and Martin A. Pomerantz "Contact Potential Differences" p. 240 & p. 241
(7)Herbert H. Ublig, 'Volta Potentials of the Copper-Nickel Alloys and Several Metals,In Air Dec. 1951 p. 1400
(8)Lord Kelvin. Phil. Mag. Vol 46 p. 82 (1895)
(9)R. Kohlrauzch. Pogg. Ann. Vol 82 p 1 (1851)
(10)W. A. Zlzman. Rev. Sec. Instr. Vol 3 p. 368 (1932)
(11)Same as (7) pp 1400
(12)Harry C. Burbridge the Thermal Coefficient of Contact Electrometer Force p. 196
13)Albert E. Hennings A..Study of Contact Potentials and Photo Electric Properties of Metals in Vacuo and the Mutual Relations Between these Phenomena
(1 4) Same-as (13) p 235
(15)H. Greinacher. Ann. d. Phys; Vol. 16, p 708 (1905)
(16)Same- as (15)
(17). P. E. Chmart A Method of Producing an Electric Current from Radioactivity Sept. 5. 1951
(18)Leland L. Antes Norman. Hacbermcrn Contact Potential Variations on Freshly Condensed Metal Films at Low Pressures. May 7. 1951
(19)Same an 17 p. 1505
Richard R. ANNIS
3 July 1952
Previous Page


